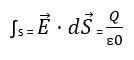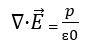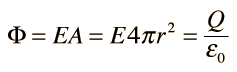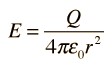Atomic models
An atomic model is an atom, the smallest unit that makes up a chemical element. Depending on whether it has a positive, negative, or neutral charge, we can find protons, electrons, or neutrons.
The atomic model was created as the years went by with the passing of historical moments and the knowledge provided by the different scientists that we will see later on. An atomic model is a representation by which we can see the structure of the atom, which allows us to know the behavior and properties of an element.
These models aim to facilitate the study of material, taking the theory of atoms to a graphical representation that is easier to understand.
How many atomic models are there?
Chronologically speaking, there are 5 atomic models, which have been updated over time:
- Dalton's atomic model (1808)
Dalton's atomic model was the first model. Dalton said that atoms cannot be split into smaller particles. Molecules are made up of several tomes. Also, when a chemical reaction takes place, the atoms of the element are rearranged. In addition, Dalton said that all the atoms that were part of an element are the same.
- Thomson's atomic model (1904)
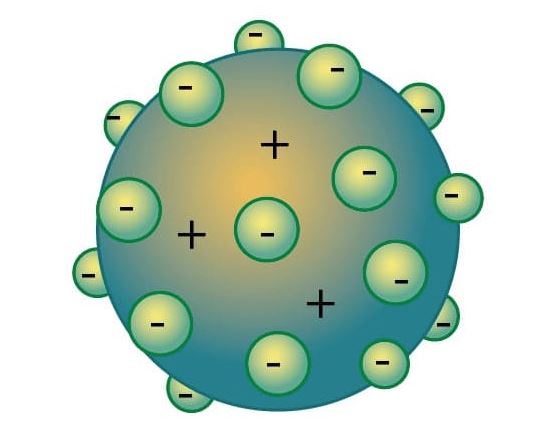
Thomson's atomic model thought that atoms were neutral. Thomson thought that atoms had passive charges in the form of protons, which were surrounded by electrons (negative charges).
- Rutherford's atomic model (1911)
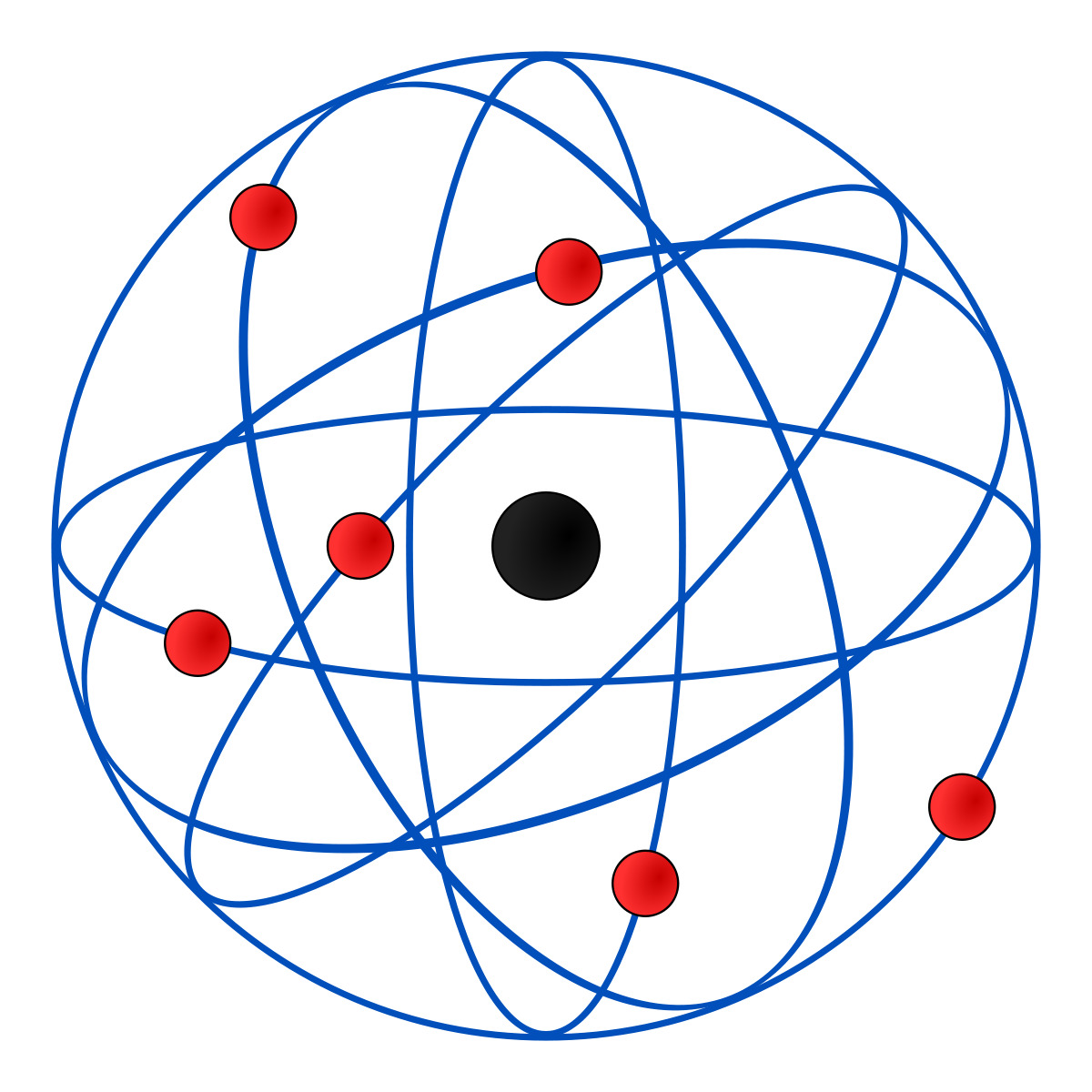
Rutherford explained that an atom has a nucleus made up of positive charges surrounded by negative particles around the nucleus. Atoms according to Rutherford were empty inside.
- Bohr's atomic model (1913)
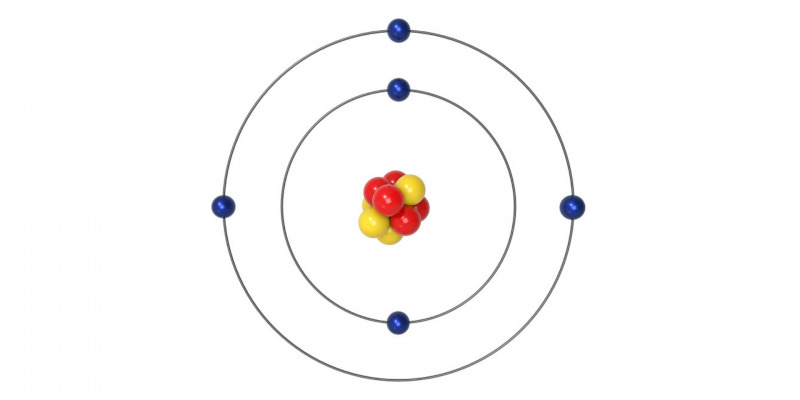
Niels Bohr proposed the representation of an atom as a likeness of the solar system with the electrons beingthe planets and the positively charged nucleus being the Sun.
- Schrödinger atomic model (1926)
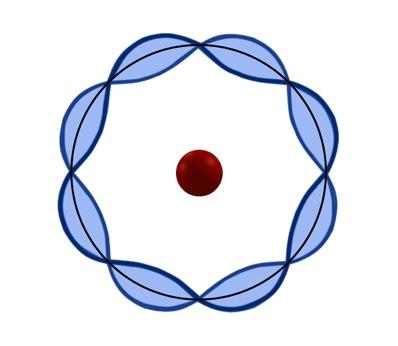
This model explains the equilibrium found between molecules and chemical bonds. Here we find electrons with different energy levels depending on the presence of the magnetic and electric field.
The current atomic model was developed by Schödinger and Heisenberg in 1920. The current atomic model is a mathematical model, where the electrons are in energy levels and have a wave-like motion.
Kirchhoff's Law
Kirchhoff's law was stated in 1824 by Gustav Robert Kirchhoff, a German physicist who specialized in electrical circuits. The law of circuits or also known as the law of meshes has the possibility of being derived from Maxwell's equations.
These two Kirchhoff laws are the same laws that rely on the charge found in electrical circuits as well as the principle of energy.
Kirchhoff's law is composed of two laws:
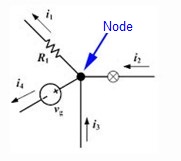
- The law of nodes or currents = the sum of the currents at a particular node (junction point of two or more conductors) is equal to the combined sum of the outgoing currents.
Conservation of charge: ∑I in = ∑ I out.
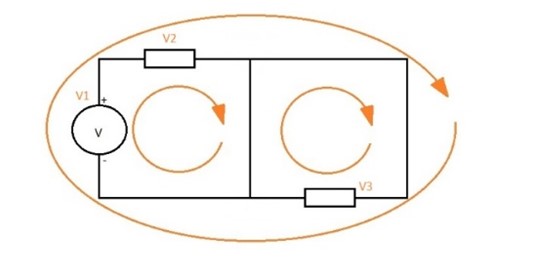
- The law of circuits or voltages= The law of circuits can also be called the rule of grids or voltages. This law states that the algebraic sum of the voltages in a closed circuit is equal to 0.
Conservation of energy: ∑ fem = ∑ (IR )
Although there are officially 2 laws, because of the relationship found in the difference between current and attention.
Node law
i2 + i1 = i3 + i4
Circuit law
V1 + V2 + V3 + … Vn = 0
Advantages of Kirchhoff's law
Kirchhoff's law allows us to calculate currents and voltages easily. On the other hand, the simplification and analysis of closed circuits can be modified.
Applications of Kirchhoff's law
The two laws are used in electrical engineering to obtain the currents and voltages at a given location in an electrical circuit.
Kirchhoff's equation gives us the possibility to calculate the enthalpy increase at different temperatures. Depending on the temperature we can find different types of enthalpy such as enthalpy of formation, enthalpy of decomposition, enthalpy of combustion, and enthalpy of neutralization. The calculation of enthalpy is an equation that is important for thermodynamics.
According to Kirchhoff's law of thermal radiation, it is determined that the emittance, as well as the absorptivity of a surface, is equal.
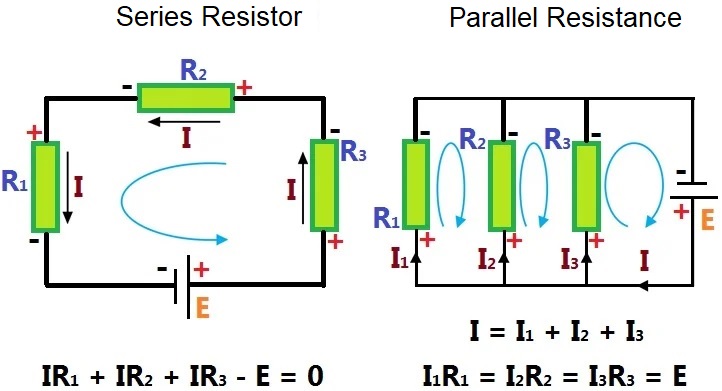
To determine the sum of n resistors by Kirchhoff's law we can find series and parallel resistors.
In series resistors, they are connected one after the other, as shown in the picture, so that if one of them stops working, the others do too. On the other hand, in the parallel resistor they have the same input, but different output; so if one stops working the others are not affected and continue working.
IMA renews the Innovative SME Seal of Approval
Ingeniería Magnética Aplicada S.L.U. has renewed the Innovative SME Seal of the Ministry of Economy, Industry and Competitiveness.
An "Innovative SME" or "R&D&I intensive SME" is considered to be an SME that in recent years has carried out activities in the field of Research, Technological Development, or Technological Innovation (R&D&I).
IMA is registered in the register of innovative SMEs in the electronic headquarters of the Secretary of State for R&D&I according to the resolution of the General Secretariat for Science and Innovation since September 2018.
Having the Innovative SME Seal means that Ingeniería Magnética Aplicada is recognized as an accredited innovative company because it carries out activities in the field of research, technological development, or technological innovation (R&D&I).
According to a report by EU experts on the Spanish R&D&I system, and aEuropean Research Area Committee (ERAC) peer review of the Spanish Research and Innovation System, there is a lack of innovative small and medium-sized enterprises. In this sense, IMA helps to reduce this gap as we are committed to R&D&I among our main activities. Furthermore, IMA is growing in line with the needs of the Spanish economy, which must move towards the incorporation of innovation in the systematic activity of companies.
Weber's theory
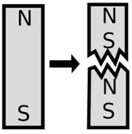
Weber's theory, also known as the molecular theory of magnets, states that a magnet can split into an indefinite number of parts. These parts do not lose the properties of magnets and maintain their magnetic poles.
In addition to this, it also states that each magnetic molecule is made of a small magnet known as a molecular magnet. On the other hand, the process of magnetization is about aligning the molecular magnets to form magnetic rows, which are called magnetic fillets, the ends of which are located at the poles formed.
Weber's theory was discovered by Wilhelm Eduard Weber, a German physicist. Weber studied terrestrial magnetism and was the inventor of the electromagnetic telegraph and an electrodynamometer. He then elaborated the molecular theory of magnets, which was later refined by Langevin.
Principles of Weber's theory
The force possessing a north and a south pole is the same. On the other hand, at the moment the substance detected in the materials is magnetized. The molecules align themselves so that the south pole points in one direction and the north pole points in the opposite direction. This alignment of the molecules is known as the substance being saturated with magnetism.
Conversely, if the magnets are not magnetized, the molecules are not aligned so they are randomly oriented, creating a closed loop.
If we look at the electrons from the point of view of the electrons, they rotate and orbit like the earth. This spinning creates a magnetic field, depending on the direction of rotation there will be one direction of magnetism or another.
As soon as a magnet is struck, the molecules begin to vibrate rapidly, releasing the attraction fields that are located at the poles, forming a closed group, and as a consequence, the magnetism weakens. Another way to lose or weaken the magnetism of the magnet is to subject it to high temperatures, the movement of the molecules goes faster, as well as the kinetic energy it produces and the same as the other way of demagnetization. The clusters close, weakening the magnetism. During the demagnetization of the magnet, the ability of the magnet to retain the residual magnetic field from the moment the magnetization is absent from the magnet will appear, this is known as retentivity.
What is spectroscopy?
Spectroscopy was discovered in 1666 by Isaac Newton who used the word spectrum to refer to all the colors that form part of the rainbow, which are obtained by passing light from the sun through a prism. Subsequently, this gave rise to spectroscopy, which is known as the study of the amount of light in an object through the interaction of electromagnetic radiation with matter. In other words, it studies spectra (the graphical representation of radiation in the matter).
In spectroscopy we can find two types of spectra:
- Absorption spectroscopy: this is a phenomenon induced by the presence of photons.
- Emission spectroscopy: these atoms at room temperature are in a fundamental state.
The study of spectroscopy deals with the breakdown of light and the measurement of the different wavelengths, whether visible or non-visible light.
One of the most common non-ionizing electromagnetic radiations is that which measures infrared radiation. Infrared spectroscopy is spectroscopy in this area. Infrared radiation is often used in the food industry to analyze the quality of food.
Processes found within spectroscopy
The interaction found between radiation and matter is spectroscopy. This interaction encompasses 3 kinds of processes:
- Absorption of radiation = in this process, one passes from a fundamental state to an excited state.
- Emission of light = here the reverse process of radiation absorption takes place, producing a photon (a particle of light energy).
- Scattering = once the matter has been bound the radiation changes direction. In addition, scattering can be of two types:
- Elastic scattering = does not produce any change in the photon.
- Inelastic scattering = does produce a change in the photon.
Atomic spectroscopic methods= this type of medical method is characterized by absorption and mission spectra, unlike the molecular method, which is only capable of absorption spectra.
Mass spectrometry is a method of separating a molecule. In the same way, we can know the molecular weight. In the same way as normal mass spectrometry, it can be divided into atomic mass spectrometry and molecular mass spectrometry. In addition to this type of spectrometry, we can also find ultraviolet and visible spectroscopy, which is the measurement of the absorption of electromagnetic radiation between the ultraviolet and the visible region.
Magnetic separation methods
Los métodos de separación magnética son unos métodos que se empezó a usar durante la segunda guerra mundial para la chatarra. Más tarde en 1970 se introdujeron 3 formas de separación: los imanes elevados, el tambor y las poleas magnéticas.
Magnet separation or also known as magnetization is a method used to separate substances where one element of the two combining substances of the heterogeneous mixture possesses magnetic properties.
Magnetic separation of heterogeneous mixtures by magnetization uses the magnetic susceptibility of one of the magnetic components. This technique consists of attracting magnetic materials, which have ferromagnetic components, with the help of magnetism and a magnet on the mixture, in this way the magnet will attract the metallic particles and separate them from the non-metallic component.
Depending on the magnetic susceptibility, minerals can be paramagnetic, ferromagnetic, or diamagnetic.
Magnetic separation has two applications: the removal of iron particles from a mixture and the separation of magnetic elements from non-magnetic elements (homogeneous mixture). To use this separation method, the homogeneous mixture must have the following characteristic: One of the components must have integrated magnetic properties.
Factors in the separation of magnetic substances
Magnetic separation depends on 3 factors:
1. Magnetic force → is reflected in the number of gausses.
2. Gravitational force → this force refers to the distance between the centers of gravity, of the particles.
3. Forces of attraction → this force is what we see when we bring the magnet closer to the mixture.
Magnetic separation uses the magnetic properties of minerals, which is why depending on the type of minerals we want to separate, we must use a magnet with more or less Gauss:
- 500 - 5,000→ strongly magnetic (magnetite).
- 5.000 - 10.000 → moderately magnetic (garnet).
- 10,000 - 18,000 → weakly magnetic (hematite).
- 18,000 - 23,000 → poorly magnetic (bornite).
Applications of magnetic separators
Magnetic separators can be used in various applications such as iron blending, iron ores, scrap recycling, conveyor belts, water cleaning...
Magnetic separation can be found in various industries such as:
- Recycling industry → we can find them in the recycling process to classify objects according to the material.
- Food industry → they are used to obtain quality products free of impurities.
- Mining and quarrying industries → where are used for the extraction of iron.
Gauss's law
Gauss's law or also known as Gauss's theorem was discovered by Karl Friederich Gauss, a mathematician who formulated in 1835 the relationship found between the electric flux circulating on a closed surface which has an electric charge. It was not until 1869 that it was published.
Gauss's law states that the electric field flux found on the closed surface is the same between the charge inside the surface and the permeability of the vacuum.
What are the properties of Gauss's law?
The properties of Gauss's law:
- The electric flux is directly proportional to the net charge of the closed surface.
- However irregular the electric flux is, it is independent of the shape of the surface.
- This electric flux does not depend on how the charge inside the surface in question is distributed.
- The number of lines passing through the surface is 0, so the external charges contribute nothing to the electric flux.
The formula for Gauss's law is?
To obtain the total electric charge we need to use the following formula
The vector of E = electric field
d together with the vector of S. = surface vector
Q = charge
![]() ɛ0= permeability of the vacuum, 8.85pFm-1
ɛ0= permeability of the vacuum, 8.85pFm-1
In addition to this formula, we can find it by the differential formula. This differential formula is obtained from the divergence theorem.
p= volume charge density
∇ = divergence
As mentioned above, the flux of the electric field on a closed surface is proportional to its charge.
The law in the form of a sphere
To obtain the magnetic field that a sphere possesses, we must use Gauss's law. This law shows us that the electric field that the sphere has is equal to the magnitude regardless of where it is on the surface.
Unlike the normal Gauss's law, when we are applying it to a sphere, to find out the magnetic field that a sphere has, we only have to multiply the magnetic field strength (the one that is on the surface of the sphere) and multiply it by the area of its surface, obtaining the following formula:
To obtain the electric field at the radius, it must be obtained using the following formula:
On the other hand, when we want to apply another charge, we must add it to the upper part by multiplying the other in the upper part of the formula.
Electromagnetic induction
Electromagnetic induction was discovered by Michael Faraday in 1831 by demonstrating the ability to generate an induced electric current from a magnetic field. This discovery led to the construction of generators.
What is electromagnetic induction?
Electromagnetic induction is the generation of electricity by the creation of electric currents through fields that change over time. In other words, the production of this electricity is obtained by magnetism.
When the magnetic field flux, in other words, the number of magnetic field lines in an electric current, is altered. According to the International System, the magnetic field flux is represented by (Wb).
The magnetic flux is represented by Φ and the number of lines passing through the surface can be calculated:
Φ = (N) · B ·S · -cos (α)
Where:
- N = Number of turns
- B = Magnetic field represented in Teslas
- S = Surface area represented in m2
The conservation of energy of electromagnetic induction is given by Lenz's law which states that the magnetic field is in the opposite direction to the flux. In addition to Lenz's law, we can find Faraday's law.
Electromagnetic induction is reflected in Faraday's law and Lenz's law. Currently, these two laws are based on and explain electromagnetics. Unlike Lenz's law, Faraday's law states the variation of magnetic flux concerning time. The voltage in the electric circuit is directly proportional to the change in the time at which the flux passes through a surface.
What is electromagnetic interference?
Electromagnetic interference is the same as electromagnetic induction except for the small difference that instead of a magnet an electromagnet or a stable magnet is used. Electromagnetic induction sensors are used in different areas such as science and geophysics.
There are two groups:
Intentional = this type of group is characterized by consciously emitting signals emitted with the purpose of creating interference.
Unintentional = they are a group of electromagnetic interference that produces interference accidentally and as a consequence obtains an unintended effect.
In addition to this classification, we can also find:
Radiated interference = an interference is considered to be radiated when the signal propagates through the air between the source and the victim via electromagnetic radiation.
Conducted interference = this type of interference occurs when the interference is propagated through the common relationship of the transmitter of the interference and the receiver. They propagate through a cable.
Joule's Law
Joule's law was a phenomenon discovered by the British James Prescott Joule, in 1840 he published his study known as "heat production by voltaic electricity" which consequently established the fact.
What does Joule's law consist of?
The current produces heat through the resistance of the conductor material. This resistance is created by the movement of electrons.
The electric charges that are produced, having a certain mass and velocity, produce kinetic energy. This energy is released when the charges collide with the atoms of the conductor. It is when this effect is in a closed circuit flowing through the current that the joule effect occurs.
How can we know the amount of heat using Joule's law?
Joule's law states that the heat that is produced by the process of an electric current is directly proportional to the resistance, the time and the intensity squared. From this we obtain the following formula:
Q=I2×R×t
- Q = the amount of heat, i.e. the Joules
- I = current intensity
- R = electrical resistance (Ω)
- t = time in which the current flows expressed in seconds.
The heat generated by the effect can be obtained from
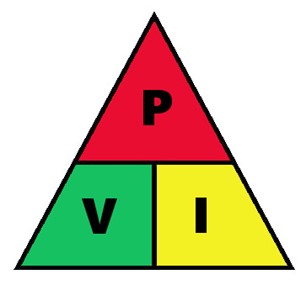
Joule's law consumes a power that is in a current. To know the power consumed, we can obtain it using the following formula:
P = V · I
Like OHM's law, we have a triangle that helps us to remember the formula, this is known as Joule's triangle. To get some magnitude we just have to cover it and from this formula, we will get the formula.
How does the law affect electricity?
When electric current heats up, it has the following effects:
- The more resistance the more heat
- The longer the period the current flows, the hotter it gets.
- The more current the more heat.
Applications of the law
Joule's law can be easily found in household appliances that generate heat, such as heaters and ovens. In these cases, they generate thermal energy as soon as the energy flows through the conductors.
This law can be found in various applications today, not only in our homes but also in the streets, for example:
- Street lamps
- Electromagnetic radiation