What is diamagnetism?
Diamagnetism is a characteristic that a magnet possesses when the magnetization in the opposite direction to the application of the magnetic field is weak. Consequently, we can observe that material with this property, in other words, a diamagnetic material, is repelled by a magnet.
In 1778, Sebald Justinus Brugmans was the first to observe that two materials (bismuth and antimony), put up resistance to being attracted to magnetic fields.
Diamagnetism was discovered by Michael Faraday in 1845, with the help of the Faraday experiment, with a negative result (so this leads directly to Lenz’s law). The acceleration of the external magnetic field slows down the electrons, so they oppose the action of the external field, weakening it.
Properties of diamagnetism
Diamagnetism, like other types of magnetic materials, has several properties:
Diamagnetism can be in liquid, solid, or gaseous form.
The relative magnetic permeability of less than 1.
Its magnetic induction and susceptibility are negative.
Diamagnetic materials are notaffected by temperature.
Once the external field is removed, the material has no ability to retain magnetic properties.
How can you tell if a material is diamagnetic or not?
To find out if a material is diamagnetic or not, we have to look at its electronic configuration, more specifically whether the electrons are unpaired or not. If the electrons are unpaired, the material will be paramagnetic, and if the electrons are paired, it will be diamagnetic.
The electronic configuration is the way in which the electrons are distributed within an atom, in which there are different layers. There are 7 energy levels: from 1 to 7, within each level, we can find 4 sublevels: s, p, d, and f.
Each sublevel has a maximum number of electrons.
| Sublevel | Nº electrons |
| Sublevel s | 2 electrons |
| Sublevel p | 6 electrons |
| Sublevel d | 10 electrons |
| Sublevel f | 14 electrons |
Examples of diamagnetic elements:
The best known diamagnetic materials are bismuth, helium, hydrogen, noble gases, gold, copper, bronze…
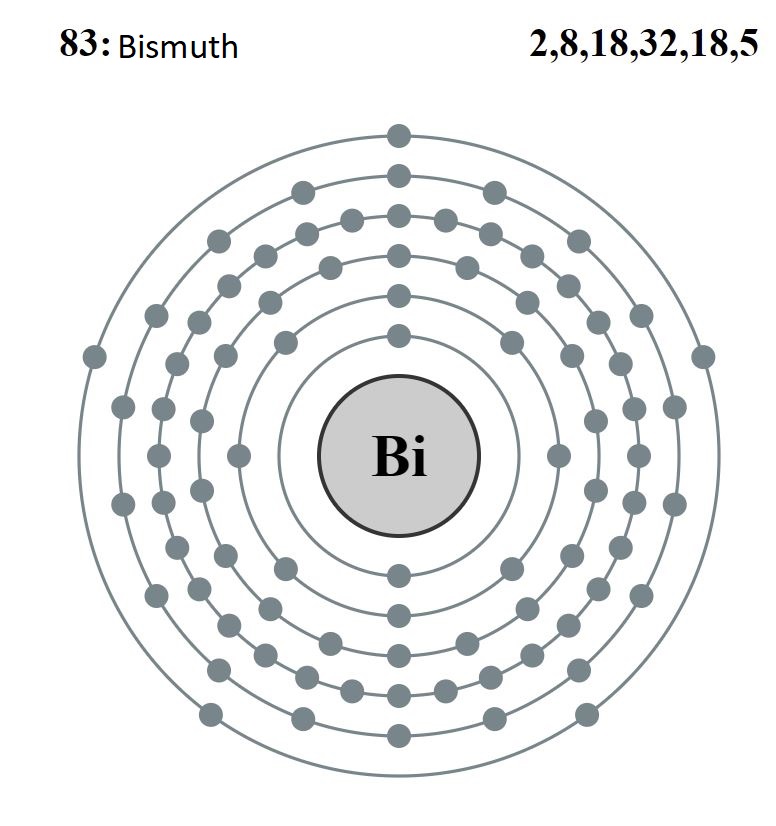
For example, bismuth has the following electronic configuration:
Bi = 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p3
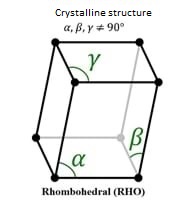
Knowing that the atomic number is 83 and it is in group 15. Below we can see the atomic structure and the crystal structure.