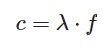
The electromagnetic spectrum
The magnetic spectrum was discovered by Wilhelm Conrad Röntgen in 1895 and was responsible for producing electromagnetic radiation in the wavelengths that refer to X-rays. Before the electromagnetic spectrum, we can find the normal spectrum which was discovered by Isaac Newton in 1666, placing several lenses next to a prism and through it, he could see the different colors.
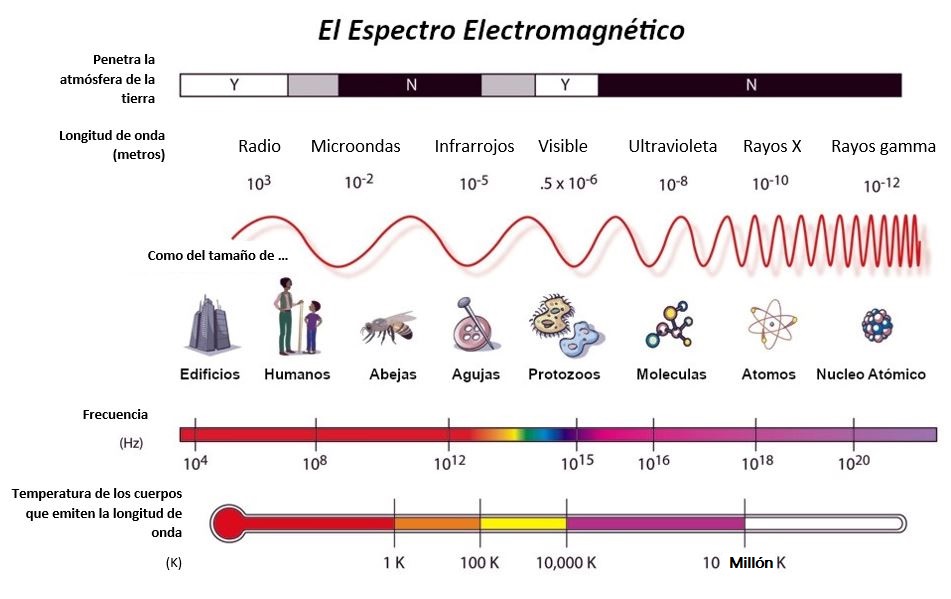
The electromagnetic spectrum is the grouping of all the forms in which radiant energy is found. This grouping of frequencies or lengths can be broken down into electromagnetic radiation. These two quantities are in relation to the speed of light.
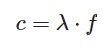
C = constant, that is to say 3.00 x 108 m/s
λ = wavelength
f= frequency
Depending on what we want to obtain, we just have to isolate the letter.
Types of electromagnetic radiation
Depending on the type of electromagnetic radiation, the electromagnetic spectrum can be divided into two groups:
Ionizing radiation:
- X-rays= longer wavelengths, found before ultraviolet radiation. For example, they can be found in the medical sector, more specifically in cancer treatment.
- Gamma rays = shorter wavelengths and higher frequencies. For example, this type of electromagnetic spectrum can be found in nuclear energy.
Non-ionizing radiation:
- Radio waves = the frequency found in this type of electromagnetic spectrum is the lowest. For example Internet.
- Microwaves= their frequency ranges between 300 MHz and 300 GHz. For example telephone, mobile phone or microwave.
- Infrared radiation= is the most important natural source of the sun, which transmits heat to us. For example heating.
- Visible light= magnetic spectrum detectable by humans. For example torches.
- Ultraviolet = its radiation takes place between visible light and ultraviolet radiation. This type of electromagnetic spectrum is absorbed by plants because it is the energy responsible for photosynthesis.
Depending on the properties of the light waves, we can find different colors such as:
| Colour | Wavelength in 10-9m | Frequency (1012Hz) |
| Red | 780 – 622 | 384 – 482 |
| Orange | 622 – 597 | 482 – 503 |
| Yellow | 597 – 577 | 503 – 520 |
| Green | 577 – 492 | 520 – 610 |
| Blue | 492 – 455 | 610 – 659 |
| Violet | 455 – 390 | 659 – 769 |
Applications of the electromagnetic spectrum
Nowadays we can find the electromagnetic spectrum in a very common way. Some of the most common applications we can find are telecommunications, information transmission, radars…