
Atomic models
An atomic model is an atom, the smallest unit that makes up a chemical element. Depending on whether it has a positive, negative, or neutral charge, we can find protons, electrons, or neutrons.
The atomic model was created as the years went by with the passing of historical moments and the knowledge provided by the different scientists that we will see later on. An atomic model is a representation by which we can see the structure of the atom, which allows us to know the behavior and properties of an element.
These models aim to facilitate the study of material, taking the theory of atoms to a graphical representation that is easier to understand.
How many atomic models are there?
Chronologically speaking, there are 5 atomic models, which have been updated over time:
- Dalton’s atomic model (1808)
Dalton’s atomic model was the first model. Dalton said that atoms cannot be split into smaller particles. Molecules are made up of several tomes. Also, when a chemical reaction takes place, the atoms of the element are rearranged. In addition, Dalton said that all the atoms that were part of an element are the same.

- Thomson’s atomic model (1904)
Thomson’s atomic model thought that atoms were neutral. Thomson thought that atoms had passive charges in the form of protons, which were surrounded by electrons (negative charges).
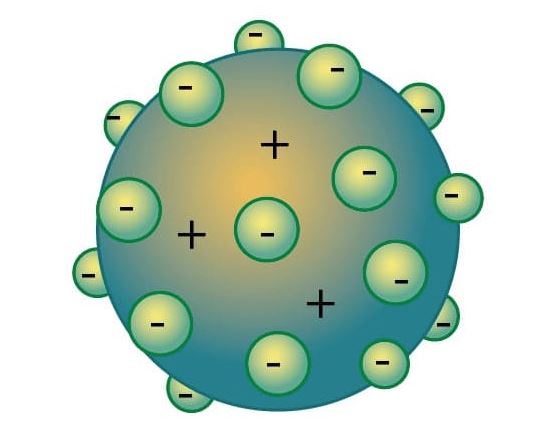
- Rutherford’s atomic model (1911)
Rutherford explained that an atom has a nucleus made up of positive charges surrounded by negative particles around the nucleus. Atoms according to Rutherford were empty inside.
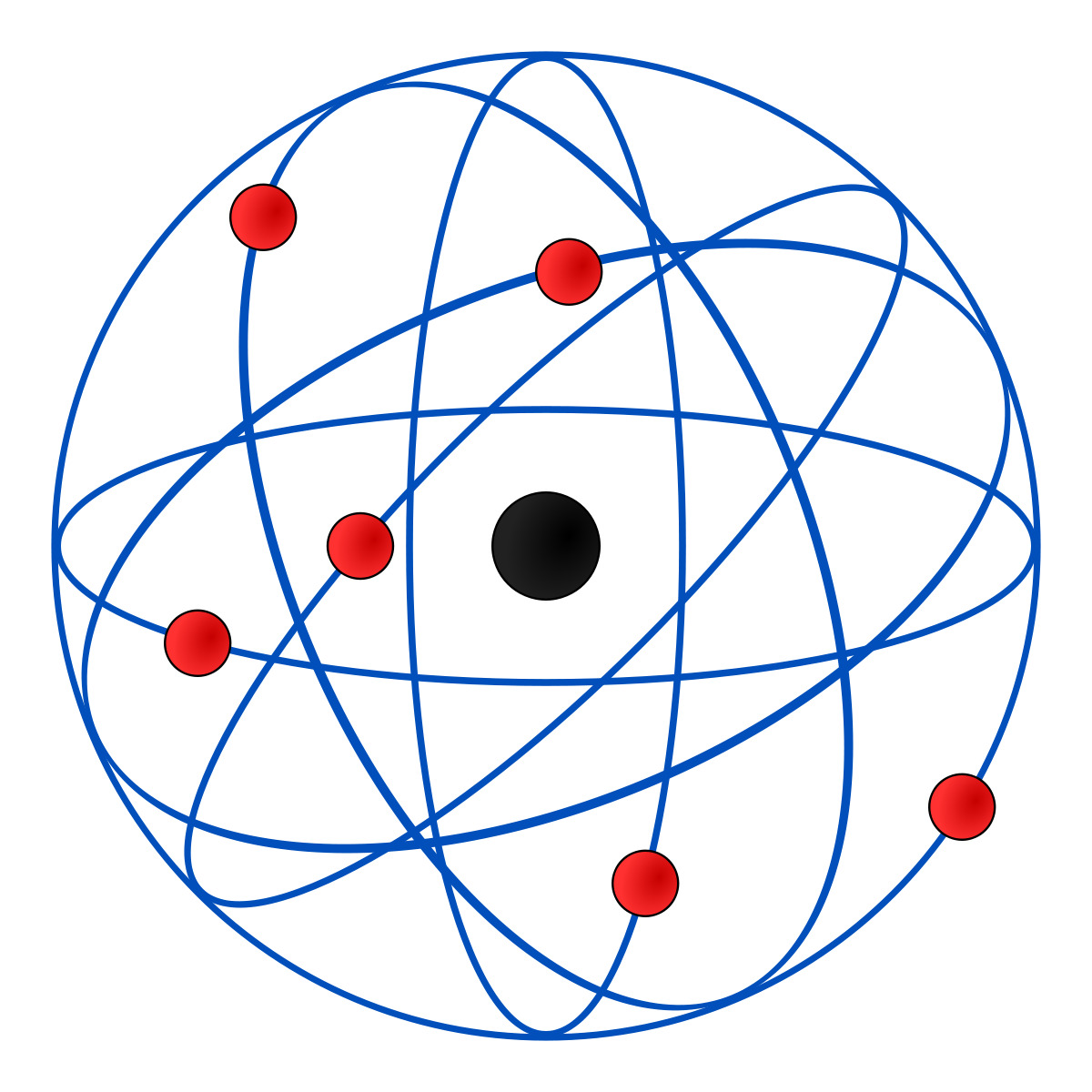
- Bohr’s atomic model (1913)
Niels Bohr proposed the representation of an atom as a likeness of the solar system with the electrons beingthe planets and the positively charged nucleus being the Sun.
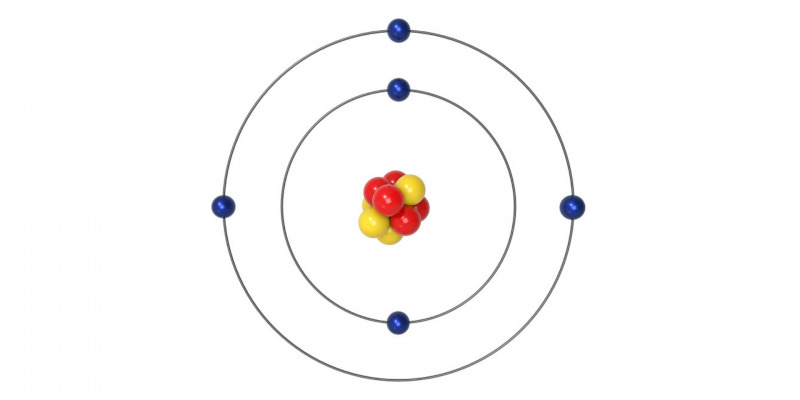
- Schrödinger atomic model (1926)
This model explains the equilibrium found between molecules and chemical bonds. Here we find electrons with different energy levels depending on the presence of the magnetic and electric field.

The current atomic model was developed by Schödinger and Heisenberg in 1920. The current atomic model is a mathematical model, where the electrons are in energy levels and have a wave-like motion.